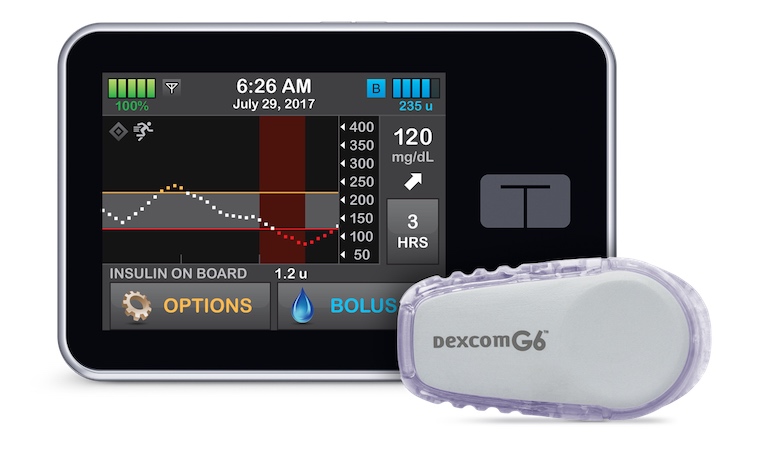
An artificial pancreas pioneered here at UVA is more effective than existing treatments at controlling blood sugar in people with type 1 diabetes, a new study in the prestigious New England Journal of Medicine has found. That sets the stage for the device to go to the federal Food and Drug Administration for approval, which would make it available to patients.
The artificial pancreas is designed to relieve people of the need to stick their fingers several times daily to check their blood sugar levels. Instead, the device does that automatically, and it administers insulin as needed. It’s a glucose monitor and an insulin pump all in one.
The new study evaluated the artificial pancreas’ effectiveness when worn by participants during regular daily life. A total of 168 participants, 14 or older, were randomly given either the artificial pancreas system or sensor-augmented pump therapy with a glucose monitor and an insulin pump that did not adjust insulin automatically.
The artificial pancreas system significantly increased the amount of time users’ blood glucose levels were in the target range of 70 to 180 mg/dL, while the other group saw no change. The artificial pancreas users also saw improvements in the amount of time spent with high and low blood glucose, hemoglobin A1c and other important measures. There were no severe hypoglycemia events in either group. One user in the artificial pancreas group had a problem with the equipment that delivers insulin and went into diabetic ketoacidosis. That one issue aside, the results were very positive.
“This artificial pancreas system has several unique features that improve glucose control beyond what is achievable using traditional methods,” said our Boris Kovatchev, PhD, director of the UVA Center for Diabetes Technology. “In particular, there is a special safety module dedicated to prevention of hypoglycemia [low blood sugar], and there is gradually intensified control overnight to achieve near-normal blood sugar levels every morning.”
While the artificial pancreas was originally pioneered here at UVA, Tandem Diabetes Care is now seeking to bring it to market. Tandem has submitted the study results to the U.S. Food and Drug Administration for approval.
I’ll let you know when we hear from the FDA.