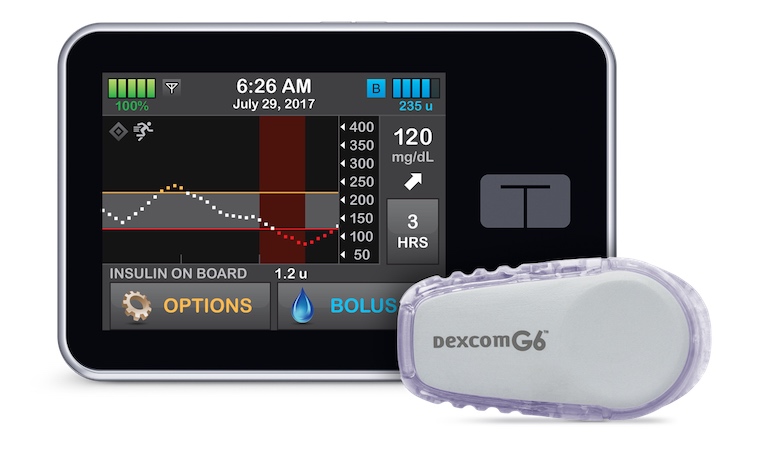
An artificial pancreas created here at UVA has been approved by the federal Food and Drug Administration. The device automatically regulates blood sugar for people with type 1 diabetes, freeing them from the need to stick their fingers multiple times each day.
The approval is a major milestone for us. The artificial pancreas has been a long time in the making, and clearing this final hurdle is a huge accomplishment for our team of researchers.
“We are excited that our decade-long research, which recently culminated in a large-scale clinical trial published in the New England Journal of Medicine, has been successfully translated to the clinical practice,” said Boris Kovatchev, PhD, director of the UVA Center for Diabetes Technology.
The artificial pancreas is being brought to market by Tandem Diabetes Care under the name Control-IQ.
What a great way to start the year. It’s worth noting it was only about a year ago that the FDA approved the use of focused ultrasound to treat Parkinson’s tremor, a technique pioneered here at UVA. How wonderful to see so much great UVA research making its way to the clinic to benefit patients everywhere.