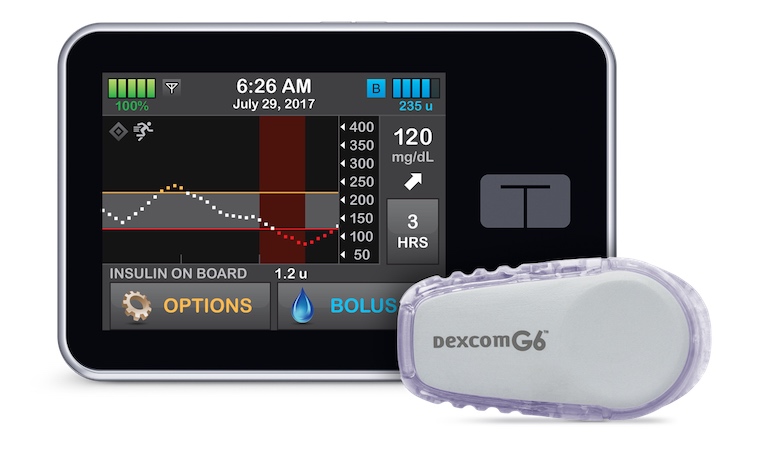
An artificial pancreas we developed at the School of Medicine is now available for children ages 6-13 who have type 1 diabetes.
A new study published in the prestigious New England Journal of Medicine found that the device, manufactured by Tandem Diabetes Care, significantly improved blood sugar control in children both day and night. This followed on previous findings that the device was effective in adolescents and adults.
The new results have prompted the federal Food and Drug Administration to approve the artificial pancreas, marketed as the Control-IQ system, for children 6-13. The device consists of an insulin pump that is programmed to automatically adjust the insulin dose as needed, based on a mathematical model using continuous glucose monitoring information.
“After the resounding success of the system in adolescents and adults in an earlier study, it is very rewarding to see younger participants in this study benefit as well, and to the same extent,” said our Marc D. Breton, PhD, the trial’s principal investigator. “We are excited to see the outcome of 15 years of research that led to these results acknowledged in the New England Journal of Medicine.”
The randomized trial enrolled 101 children ages 6 to 13 at UVA, Stanford, Yale and the University of Colorado. The children were given either the artificial pancreas or a standard continuous glucose monitor and separate insulin pump.
The artificial pancreas proved clearly superior: The average percentage of time the children’s blood sugar was in the target range during the day was 7 percentage points higher using the artificial pancreas. Nighttime control, which is particularly important, was 26 percentage points higher.
Parents who think their children might benefit from the artificial pancreas can discuss it with their pediatrician.